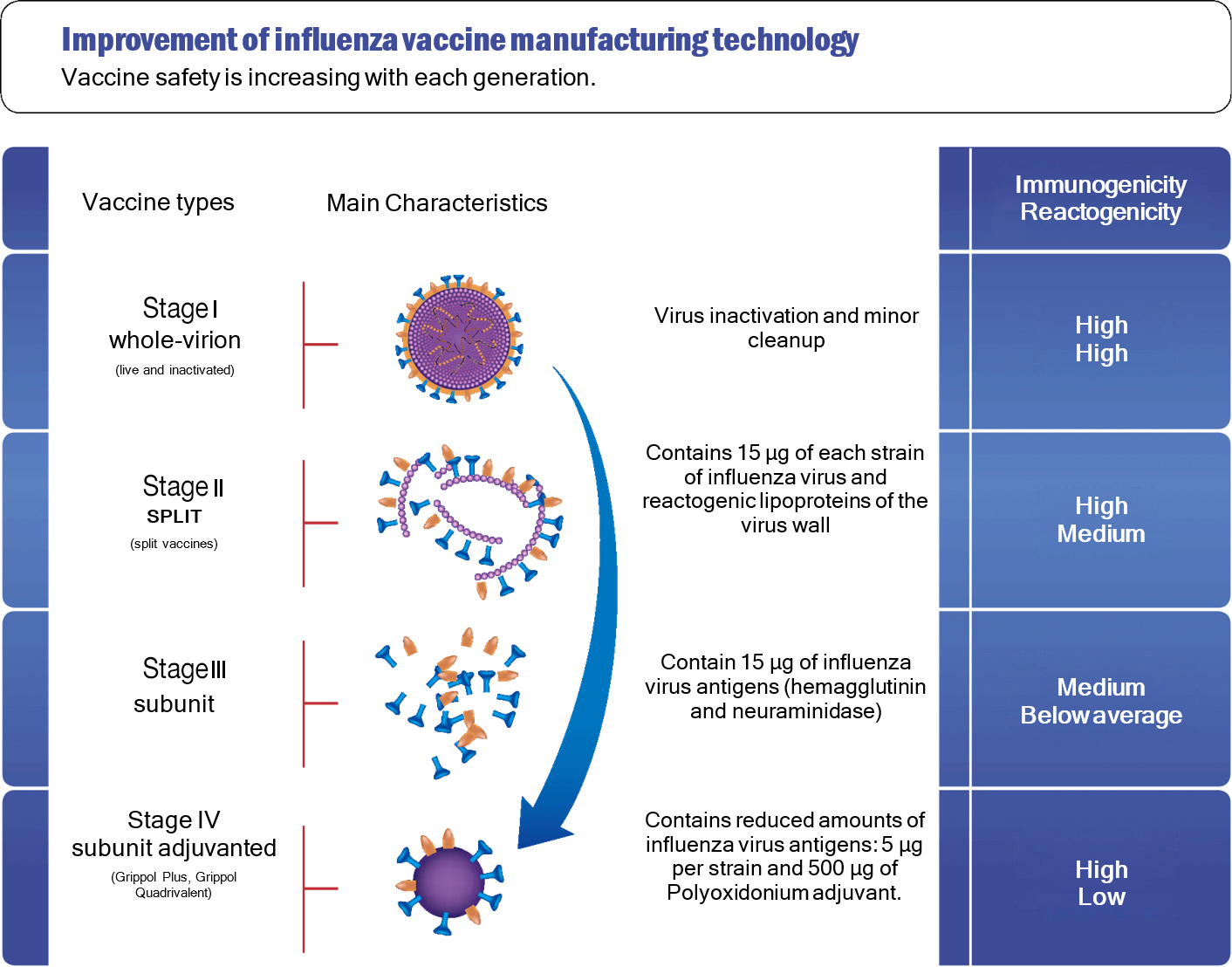
INFLUENZA VACCINE TYPES
Influenza vaccines can be divided into several generations, reflecting the stages of improvement in their
manufacturing technology.
All vaccines are comparable in efficacy, but with each generation,
their safety is improving.
There are three main stages in the development of influenza vaccines:
Stage 1. The creation of whole-virion vaccines.
These include two types of whole-virion vaccines - live and inactivated. They are influenza artificially weakened
(live vaccine) or killed viruses, i.e. inactivated and purified (inactivated vaccine).
Created back in the 1950s, whole-virion vaccines contained many components of the influenza virus, which put extra
load on the immune system. These vaccines are considered the most reactogenic, which severely limits their use,
especially in children and pregnant women. The principle behind these vaccines was that once inhaled, they simulated
infection, i.e. to become immune the vaccine would actually make a person contract influenza (albeit in a mild form)
and still be contagious to others.
The fact is that the influenza virus spreads quickly by airborne droplets and mutates easily, forming new strains
against which humans have no immunity. If different influenza viruses are present in the same organism at the same
time (e.g. avian and human influenza viruses), there is a high probability that the genetic material of the two
strains will be exchanged and a new strain with new properties will emerge. When a person is vaccinated with the
live vaccine, he/she becomes a carrier and spreader of the virus for three weeks, even if weakened, but quite
viable. Therefore, WHO experts now recommend immunization with inactivated vaccines only.
Stage 2. The creation of split vaccines
The developers of split vaccines have started using not the whole influenza virus (virion), but purified influenza
virus surface antigens and internal proteins. These vaccines contain “killed” (inactivated) influenza virus cut into
separate parts. These parts contain both antigens necessary for the body's protection against influenza and other
elements that are not necessary for the formation of an immune response - elements of the membrane, internal
proteins, RNA structures of the virus.
Split vaccines contain 15 µg of antigen of each type of influenza (A, B) of the current strains. They are highly
immunogenic, but also sufficiently reactive as they contain additional impurities of proteins of the virus interior.
The frequency of side-effects is, of course, lower than that of whole-virion vaccines and, at the same time,
split-vaccines are more reactive than subunit vaccines.
Phase 3. The creation of subunit vaccines.
Subunit vaccines are inactivated (do not contain live virus particles), but unlike split vaccines, they contain only
influenza virus antigens (highly purified surface proteins, haemagglutinin, and neuraminidase).
These vaccines also contain 15 µg of antigen per strain, but they no longer contain additional protein impurities.
Influenza virus researchers have been able to prove that it is against the surface antigens of haemagglutinin and
neuraminidase viruses that antibodies most effective in protecting against influenza are formed. The virus' internal
proteins do not have these properties. Thus, a vaccine containing only surface antigens remains just as effective,
but becomes safer when administered by reducing side-effects.
With the advent of subunit vaccines, influenza immunoprophylaxis has become even more effective and safer and the
frequency of side effects has decreased. In addition, subunit vaccines were used for the first time in patients for
whom vaccination was previously contraindicated, including highly allergic individuals.
Stage 4. The creation of subunit adjuvanted vaccines.
Influenza vaccines have evolved to become safer, with improvements in technology to remove viral material from
ballast compounds. However, WHO is still challenging vaccine developers to improve the effectiveness of vaccines
and, more importantly, their safety for all population groups. For this reason, scientists worldwide have spent the
last 15 years searching for a safe adjuvant that could enhance the immune response and increase its speed and
duration.
The inclusion of Polyoxidonium in the Grippol influenza subunit vaccine has enabled creating a new type of
vaccine (subunit adjuvanted) with high immunogenicity and the highest safety profile.
Type I-III inactivated vaccines usually contain 15 µg per haemagglutinin strain, as at a lower dose, they would not
be effective. The composition and quality of vaccines is governed by the European or National Pharmacopoeia, and the
European Pharmacopoeia specifically stipulates that the antigen content should be 15 mg, unless a different dosage
is justified by the results of clinical trials. So, the use of less than 15 µg of haemagglutinin antigen per dose of
influenza vaccine is permitted by both the European and Russian Pharmacopoeias, if clinical trials confirm the
efficacy of using a smaller amount.
The use of Polyoxidonium adjuvant in the Grippol® Plus vaccine reduces the dose of viral antigens by a factor of
three and therefore makes the vaccination safer. This unique technology has been used for more than 20 years to
produce Grippol vaccines, which are widely used in mass immunizations.
Clinical and epidemiological studies of influenza vaccines have convincingly demonstrated that a dose of 5 µg of
each strain in combination with the adjuvant Polyoxidonium is sufficient and effective, providing high immune
response and an increased safety profile by reducing the antigenic burden on the body.