GRIPPOL®
QUADRIVALENT
INACTIVATED SUBUNIT INFLUENZA VACCINE
Grippol Quadrivalent is used for vaccination children and adults aged 6 to 60 years and over.
- with chronic heart, blood, and vascular diseases;
- nervous system and brain diseases;
- respiratory pathologies, including bronchial asthma, COPD, and smokers;
- those affected by kidney disease, diabetes mellitus, and metabolic disorders, including obesity;
- persons with autoimmune, allergic diseases (except chicken protein allergy);
- sickly people;
- those affected by HIV and other immunodeficiencies;
- people who have a high risk of getting the flu or infecting others because of their occupation.
 4 STRAINS
4 STRAINS  3 TIMES
3 TIMES  QUALITY STANDARD
QUALITY STANDARD - Preservatives and Antibiotic Free
- Available in individual syringe doses with an atraumatic needle
- Produced according to international GMP standards
 > 300M DOSES
> 300M DOSES To date, only trivalent influenza vaccines have been used in the Russian Federation. In 2019, the Ministry of Health, together with Rospotrebnadzor, developed and approved a Phased Transition Plan for the use of quadrivalent vaccines to prevent influenza as part of the National Preventive Vaccination Calendar between 2019 and 2021.
Grippol®Quadrivalent contains 4 of the most common antigenic strains, which means that once it is administered and an immune response is formed, a person has a broader spectrum of protection against different types of viruses. Accordingly, the risk of contracting influenza is reduced.
| Country/ Regulatory body |
Year when the recommendation was made | Age/group of vaccinated persons |
|---|---|---|
| WHO | 2012 | Pregnant, children under 5 years old, health care professionals, elderly, persons with chronic diseases |
| Germany | 2013 | Pregnant; children under 5 years old; health care professionals, elderly; persons with chronic diseases; people planning long journeys |
| USA | 2013 | Children from 6 months and adults |
| Hong Kong | 2013 | Children over the age of 3 and adults |
| Canada | 2014 | 6 months and over |
| France | 2014 | Children over the age of 3 and adults |
| Belgium | 2015 | Over the age of 2 |
| Brazil | 2014 | Elderly over 60 years old |
| Australia | 2015 | 6 months and over |
| Great Britain | 2013 | Children 2-7 years old and children at risk 2-18 years old |
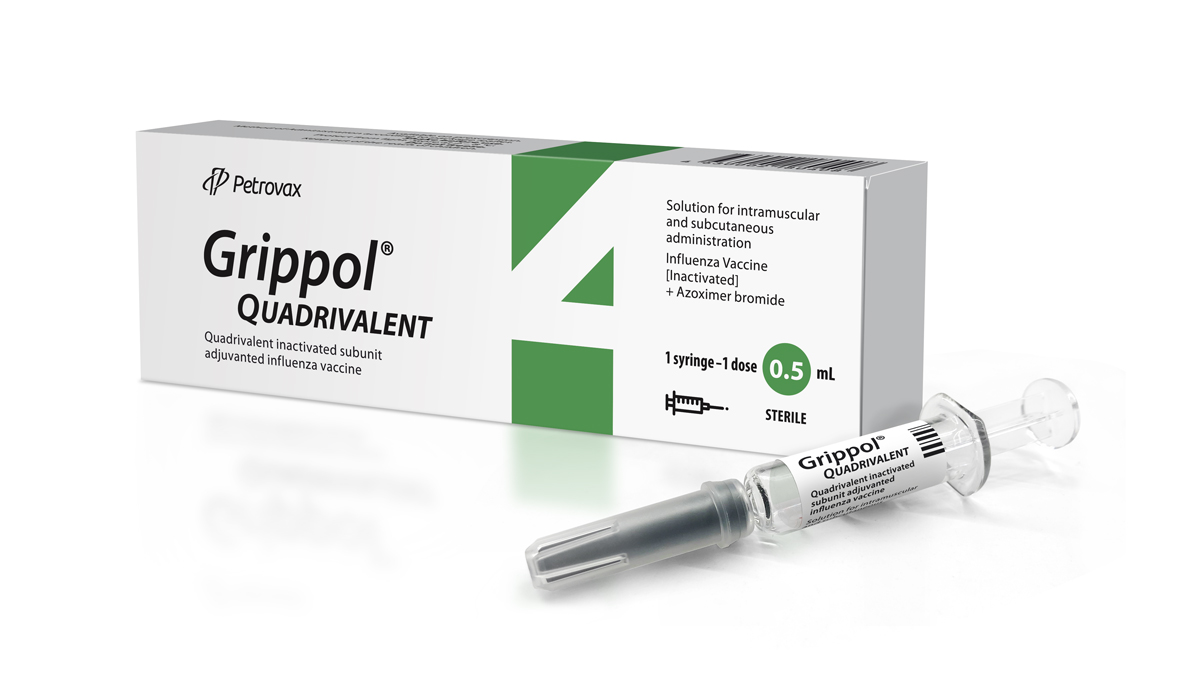
- Contains antigens of 2 influenza A subtypes (H1N1 and H3N2), 2 influenza B strains (Victoria and Yamaga), and the Polyoxidonium® immunoadjuvant
- Preservatives-free
- Available in individual syringe doses with an atraumatic needle
- Developed by Grippol® Vaccine Technology