ADJUVANTED INFLUENZA VACCINES
As defined by the FDA (Food and Drug Administration, USA), adjuvants are substances that enhance specific immune
responses to an antigen1.
They have been used for decades to improve the immune response to antigens contained in vaccines. According to the
European Medicines Agency (EMEA), the inclusion of adjuvants in vaccines aims to enhance, accelerate and prolong the
immune response to vaccine antigens 2.
Benefits of using adjuvants:
- Improve antigen immunogenicity. Due to the properties of adjuvants, it is possible to increase the efficacy of vaccines, to ensure that immunity is active without overstimulation and the risk of hypersensitivity.
- Less antigens — less risk of side effects to vaccination. The addition of some adjuvants helps to reduce the antigen load on the body: they stimulate the immune system response and a reduced dose of antigens is effective 2. Так, например, работает Полиоксидоний®, который в качестве адъюванта добавляют в вакцины в Гриппол®Плюс и Гриппол®Квадривалент.
- Time-tested. Most adjuvants have long been used in vaccine production and their doses in vaccine products are minimal. Vaccines with adjuvants based on aluminium, either hydroxide or phosphate, have long been used both in Russia and the world3. It is thanks to adjuvants that so many vaccines against topical infections have been created - most of the vaccines in the National Preventive Vaccination Calendar, not only in Russia, but also abroad.
- Improved immune response in the elderly and immunocompromised persons 2. For example, an influenza adjuvanted vaccine containing the squalene-based water-emulsion adjuvant MF59 is used worldwide. This vaccine was specially developed in Europe for older people whose immunity no longer responds so well to vaccination.
- Providing an antigen-saving strategy in an environment of limited antigen supply 3
Adjuvants & Safety
The first adjuvants were developed with the aim of increasing the antibody response, and this single requirement was
sufficient. However, the issue of post-vaccination reactions has always been of concern to patients and health
professionals. Some types of adjuvants may cause local reactions to the vaccine (redness, pain, local increase in
temperature, indurations) or general reactions (increase in temperature, hypersensitivity reactions). Generally,
they do not worsen the benefit/risk ratio of vaccination, and many vaccines simply would not work without the
addition of such components.
The safety of incorporating adjuvants into vaccines is one of the WHO's Global Vaccine Safety priorities 4, 5. Therefore, the search continues today for adjuvants that:
- Would provide sufficient vaccine efficacy without increasing the amount of antigens in the vaccine;
- would have no or minimal side-effects of their own;
- do not have an active immunomodulatory effect.
One representative of adjuvants with such properties is the Russian Polyoxidonium® (Azoximer bromide), which is
added at a dose of 500 µg to the influenza vaccines Grippol® Plus and Grippol® Quadrivalent. In the vaccine,
Polyoxidonium does not work as an immunomodulator but as an adjuvant, locally at the injection site, stimulating the
natural immune response to antigens. Azoximer bromide is listed in the FDA’s Substance Registration System77
What properties make Polyoxidonium work as an adjuvant?
The Polyoxidonium works as an adjuvant in the vaccine, performing three important functions 8:
- Increases the representation of co-stimulatory molecules on cells that recognize the introduced antigen;
- It creates conditions for effective presentation of antigens to immune cells (macrophages)
- Enhances the formation of an immune response to introduced antigens by stimulating antibody response and non-specific defense factors (dendritic cells, cytokines, etc.)
Adjuvanted formula with Polyoxidonium® in Grippol vaccines increases the safety and efficacy of vaccination
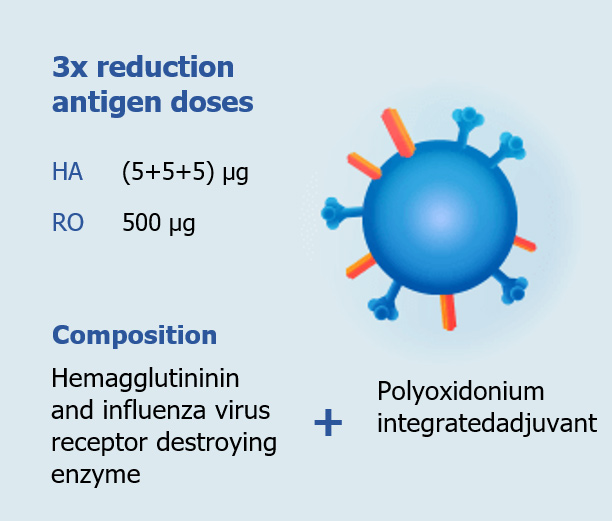
Polyoxidonium ® in the vaccine works in several ways:
- Allows decreasing the number of antigens, ensuring a pronounced and long-lasting immune response to vaccination.
- Increases the immune response development rate.
- Strengthens the immune response in immunologically immature persons.
- Reduces the protein load on the body and increases the safety of the vaccine.
In general, Polyoxidonium® as an adjuvant in Grippol vaccines allows:
- To reduce the antigen content of vaccines to 5 µg per strain of influenza virus, while ensuring a high immune response to vaccination
- To reduce the antigenic load on the body, thereby increasing the safety of vaccination by reducing the frequency of general and local reactions to its injection.
The efficacy and safety of Polyoxidonium as an adjuvant for influenza vaccines has been also confirmed by a report from FluConsult,the independent European company. The conclusions of the examination were as follows.9
- Polyoxidonium® as an adjuvant for influenza vaccines is safe and does not increase the reactogenicity of the vaccine
- A reduced antigen count of 5 µg per strain of vaccine containing Polyoxidonium® as adjuvant forms an immune response comparable to that of vaccines without adjuvant containing 15 µg of antigen per strain of influenza virus
1. Guidance for Industry for the Evaluation of Combination Vaccines for Preventable
Diseases: Production, Testing and Clinical Studies. – U.S. Department of Health and Human
Services Food and Drug Administration Center for Biologics Evaluation and Research. 1997;
2. Guideline on adjuvants in vaccines for human use//EMEA/CHMP/VEG/134716/2004.
London, 20 January 2005.
URL:http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003809.pdf;
3. Guideline on clinical evaluation of vaccines. EMEA/CHMP/VWP/164653/05 Rev. 1 //
EMA. – 26.04.2018;
4. Aluminium adjuvants. Global Vaccine Safety// WHO. – 2012. URL:
http://www.who.int/vaccine_safety/committee/topics/adjuvants/Jun_2012/en/;
5. Aluminium adjuvants. Global Vaccine Safety// WHO. – 2012. URL:
http://www.who.int/vaccine_safety/committee/topics/adjuvants/Jun_2012/en/;
6. Technology Transfer: Adjuvants. The Global Adjuvant Development Initiative. // WHO.
http://www.who.int/phi/implementation/techtransfer_vaccines_adjuvants/en/;
7. https://fdasis.nlm.nih.gov/srs/unii/90g53638zd;
Mechanisms of Polyoxidonium. adjuvanted effects //Immunology of hematopoiesis 2015; t.3: 30-92;
http://www.imhaemo.ru/media/documents/2016-02-09/Journal_HI_2015_2.pdf;
9. B. Compierre. Safety and efficacy of a subunit influenza vaccine containing Polyoxidonium. A systematic review
and meta-analysis of clinical trials//Epidemiology and Preventive Vaccination, 2018;17(4):92-98.