
RUSSIA'S FIRST TRIVALENT
INACTIVATED SUBUNIT ADJUVANTED PRESERVATIVES FREE INFLUENZA VACCINE
INACTIVATED SUBUNIT ADJUVANTED PRESERVATIVES FREE INFLUENZA VACCINE
More than 160 million doses of Grippol Plus vaccine have been used in 14 years of massive immunization
The Grippol plus vaccine composition
The Grippol Plus vaccine contains purified antigens of influenza virus circulating strains that are recommended by the WHO for the current epidemic season. One dose of Grippol® Plus contains 5 µg of haemagglutinin of each of the current strains of subtypes A (H1N1 and H3N2) and B influenza viruses, and 500 µg of the Polyoxidonium adjuvant.INFLUENZA® PLUS ADJUVANT IMPROVES VACCINATION EFFICACY AND SAFETY
An adjuvant is a substance used in combination with a specific antigen that provides
a more pronounced immune response than the antigen itself.
Polyoxidonium in the vaccine is effective in several directions:1

Allows a reduction of antigens, ensuring a pronounced and long-lasting immune response to vaccination

Increases the rate of immune response2

Strengthens the immune response in immunocompromised persons

Reduces protein load on the body
Grippol® Plus Vaccine: Indications for Use
- Children from 6 months of age
- Pregnant women as decided by their doctor (the safest vaccination is in the second and third trimesters)
- The vaccine is also indicated to
- Teenagers
- Adults with no age limit
- People at high risk of complications if they get the flu
- People who have a high risk of getting the flu or infecting others because of their occupation.
- Pregnancy and breastfeeding are not a contraindication to vaccination
The benefits of using the Grippol Plus vaccine:
High Efficacy and Safety Profile
The Grippol® Plus is designed to prevent influenza in children over 6 months of age,
pregnant women, the elderly and adults with chronic diseases, including highly allergic
individuals and those with immune deficiencies. Comparative trials of Grippol® Plus in a
ccordance with global Good Clinical Practice standards have proven that the vaccine is highly effective,
on par with the best foreign equivalents and has a high safety profile
Minimal side effects
The introduction of the Polyoxidonium adjuvant into the vaccine
formulation reduces the number of antigens while maintaining high immunological potency.
Affordable influenza prevention
Grippol Plus is produced in accordance with international GMP manufacturing standards and
is affordably priced as compared to foreign vaccines.
It could be used for combined immunization with National Preventive Vaccination Calendar vaccines (except BCG, BCG-M)
Additional safety for Grippol® plus vaccination
- No preservatives and antibiotics
- Individual syringe dose with atraumatic needle is available.
- High Quality GMP Manufacturing Conditions
- The presence of highly purified antigens produced by Abbott Biologicals
Syringe system used in Grippol plus vaccine promotes vaccination safety
- Adhesive needle with special atraumatic grinding (manufacturer - Japan). Needle lock - double layer, inner layer is high quality rubber, outer layer is plastic
- The RTF® (Ready to Fill) syringes are the latest injection system representing a glass case (Gerresheimer Concern, Germany) with a rubber piston and plastic pusher. The syringes are delivered to the factory assembled, siliconized and sterilized, i.e. fully ready for filling
- The final stage - packaging is 100% automated, while other suppliers use visual inspection.
- High-quality syringes and automatic inspection of production facilities ensure safe administration and high quality of the product
COMPARATIVE ASSESSMENT OF SAFETY, IMMUNOGENICITY
AND CLINICAL EFFICACY OF GRIPPOL® VACCINE PLUS WITH OTHER VACCINES3
Seroprotection rate (%)
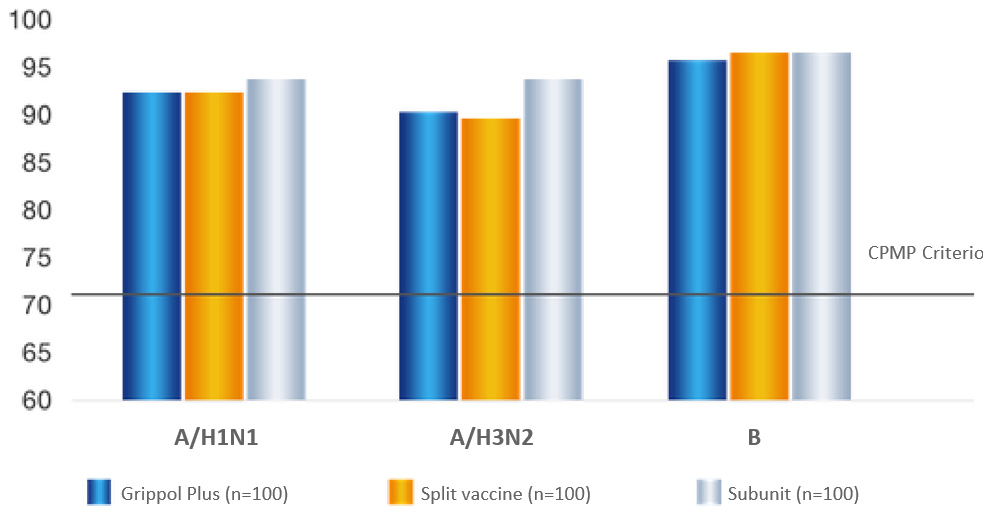
Grippol® plus provides effective protection against all three antigens of current influenza virus strains and
is comparable to the split-vaccine and subunit comparison groups.
Characteristics of the post-vaccination period (%)
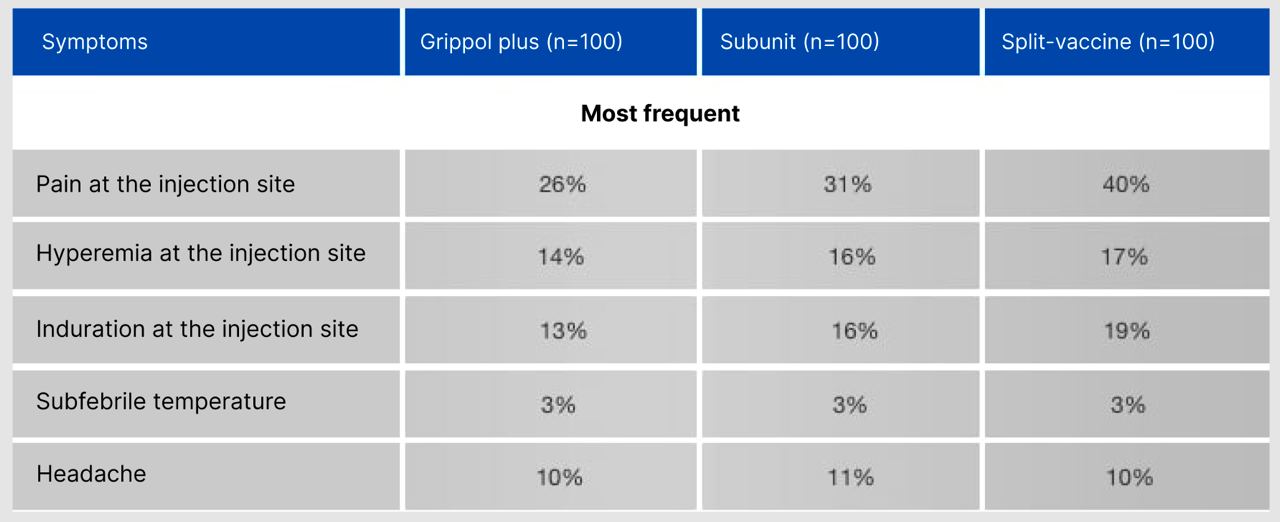
Grippol® Plus has a lower reactogenicity than split or subunit inactivated influenza vaccines.
The percentage of patients affected with any infectious disease after vaccination was: 14% in the subunit group,
11.0% in the split-vaccine group and 9.0% in the Grippol plus group.
4

1. Wojciechowska E.M. et al. Immunogenicity results of the new Grippol Plus influenza vaccine // Epidemiology and
Preventive Vaccination, №1 (44) /2009;
2. B. Compierre. Safety and efficacy of a subunit influenza vaccine containing Polyoxidonium. Systematic review and
meta-analysis of clinical trials//Epidemiology and Preventive Vaccination, 2018; 17 (4) :92-98;
3. Harit S.M., Lioznov D.A. and others, Comparative assessment of reactogenicity and immunogenicity of commercial
influenza inactivated vaccines: Grippol Plus polymer-subunit, Influvac subunit, and Vaxigrip split vaccine,
Epidemiology and Preventive Vaccination, 2017, №2 (93), pp: 24-31;
4. Study on comparative assessment of reactogenicity and immunogenicity of commercial influenza inactivated
vaccines: Grippol® plus polymer-subunit vaccine, Influvac subunit vaccine, Vaxigrip split vaccine, NIR-Gp-14-co
2014.