Grippol® Quadrivalent - quadrivalent vaccine with protection against 4 strains of influenza virus (2A+2B)
Grippol® Quadrivalent vaccine has been available in Russia since 2018. It is a modern quadrivalent vaccine that
contains antigens from 4 strains of influenza viruses and the Polyoxidonium adjuvant. It is used to immunize adults
from 18 to 60 years old.
Grippol® Quadrivalent vaccine has passed all required clinical trials, demonstrating a high level of safety and
efficacy in accordance with the EMA CPMP and Russian Federation State Pharmacopoeia criteria for influenza
inactivated vaccines.
A multicentre, double-blind, randomized, parallel-group study of 612 people aged 18-60 years evaluated the safety,
reactogenicity and immunogenicity of the Russian quadrivalent influenza vaccine1.
Clinical centers where research was conducted: FSBI “Smorodintsev Research Institute of Influenza ” of the
Ministry of Health of RF, FSBI Research Institute of Pediatric Infections (Children's Research and Clinical
Center for Infectious Diseases) of the Federal Medical and Biological Agency, Pavlov University of the Ministry
of Health of RF.
Vaccine under study: Grippol® Quadrivalent, with the following antigen composition: influenza type A virus
antigen (H1N1), influenza type A virus antigen (H3N2), influenza type B virus antigen (Yamagata strain),
influenza type B virus antigen (Victorian strain 5 μg each, and 500 μg each of Polyoxidonium® adjuvant.
Comparison vaccines:
- Group I: Grippol® Plus Vaccine containing B Strain (Yamagata strain)
- Group II: Grippol® Plus Vaccine containing B Strain (Victoria strain)
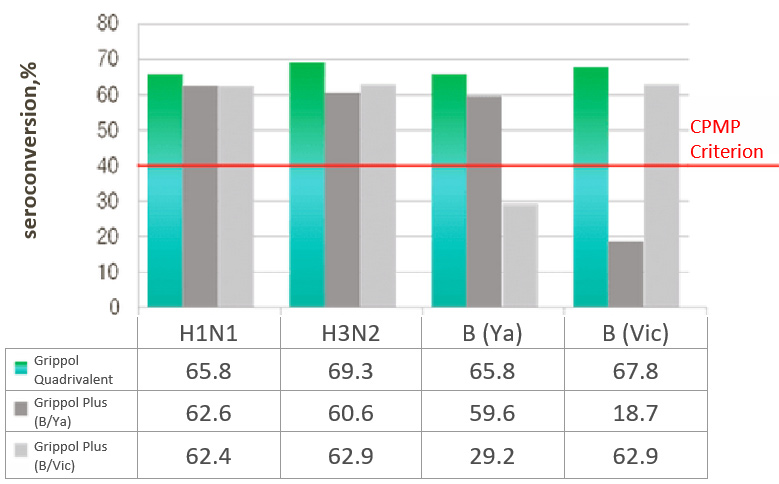
Immunogenicity of Grippol® Quadrivalent
Clinical trials showed that the vaccine met the immunogenicity criteria for inactivated influenza vaccines for all
four included strains: 1 month after vaccination of healthy volunteers, the seroconversion rates to strains A/H1N1,
A/H3N2 and B/Yamagata and B/Victoria were 65. 8%, 69.3%, 65.8% and 67.8%, and the titers fold increase was 4.9, 5.3,
5.4 and 4.8, respectively.
RESULTS OF THE INFLUENZA® QUADRIVALENT IMMUNOGENICITY ASSESSMENT IN ADULTS
SEROCONVERSION RATE,%
ANTIBODY TITERS FOLD INCREASE
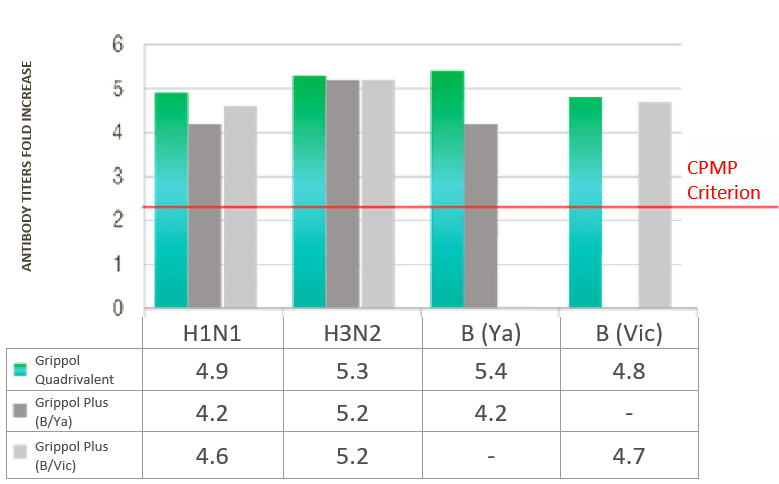
- Immunogenicity of Grippol® Quadrivalent is comparable to trivalent vaccines for matched strains and is superior for the fourth strain that is not part of the reference vaccines
- Immunogenicity of Grippol® Quadrivalent meets EMA CPMP criteria for all 4 strains of influenza virus2
- Adding the 4th strain to the Grippol® Quadrivalent provides greater protection against influenza B viruses and does not reduce the production of an immune response to other strains
GRIPPOL® QUADRIVALENT HAS A HIGH TOLERABILITY AND SAFETY PROFILE
| Most frequent local reactions | |||
|---|---|---|---|
| Grippol Quadrivalent (N=205) | Grippol Plus (V/Ya) (N=205) | Grippol Plus (V/Vic) (N=202) | |
| Pain at the site of injection | 18,5% | 11,2% | 14,4% |
| Redness (< 30 mm) | 20% | 14,6% | 18,8% |
| Swelling | 13,2% | 6,8% | 9,4% |
| Itching | 6,8% | 4,9% | 4,5% |
| Most frequent systemic reactions | |||
|---|---|---|---|
| Grippol Quadrivalent (N=185) | Grippol Plus (V/Ya) (N=187) | Grippol Plus (V/Vic) (N=187) | |
| Temperature > 37°C | 0 | 1,6% | 1,6% |
| Malaise | 3,8% | 7,5% | 5,3% |
| Headache | 4,9% | 10,2% | 6,4% |
- No significant differences in the frequency of reactions to Grippol® Quadrivalent and trivalent vaccines
- Local and general reactions occurred mostly within the first 3 days after vaccination and did not require medical intervention
The number of reactions to the vaccine did not exceed 5% for general reactions and 20% for local reactions;
the most frequent general reaction was headache (4.9%)
and the most frequent local reaction was redness at the injection site (20.0%)
1. D.A. Lioznov, S.M.Kharit, M.K.Erofeeva et al. Evaluation of reactogenicity and immunogenicity of a quadrivalent
inactivated subunit influenza vaccine.//Epidemiology and Preventive Vaccination 2018; No. 3(100):23-27.
2. Available at:
https://www.ema.europa.eu/documents/scientific-guideline/note-guidance-harmonisation-requirements-influenza-vaccines_en.pdf